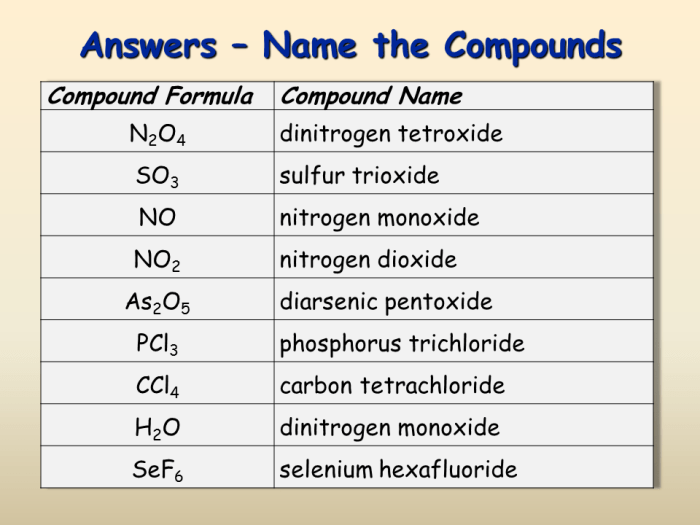
Mg h2po3 2 chemical name – MgH2PO3·2H2O, commonly known as magnesium hydrogen phosphate, is a fascinating chemical compound with a unique set of properties and diverse applications. Delving into its chemical name, we embark on a journey to explore the intricate nature and practical uses of this remarkable substance.
MgH2PO3·2H2O possesses a molecular formula of MgH2PO3·2H2O and a molar mass of 123.31 g/mol. Its physical properties include a white, crystalline appearance, high solubility in water, and a melting point of 100°C.
Basic Information
Magnesium hydrogen phosphate dihydrate (MgH2PO3·2H2O), also known as magnesium phosphate monobasic dihydrate, is a white, crystalline powder that is soluble in water. It has a molecular formula of MgH2PO3·2H2O and a molar mass of 124.31 g/mol. The melting point of MgH2PO3·2H2O is 110°C.
Chemical Name
The chemical name of MgH2PO3·2H2O is magnesium hydrogen phosphate dihydrate. It is a salt that is composed of the magnesium ion (Mg2+), the hydrogen phosphate ion (HPO42-), and two water molecules (H2O).
Molecular Formula and Molar Mass
The molecular formula of MgH2PO3·2H2O is MgH2PO3·2H2O. Its molar mass is 124.31 g/mol.
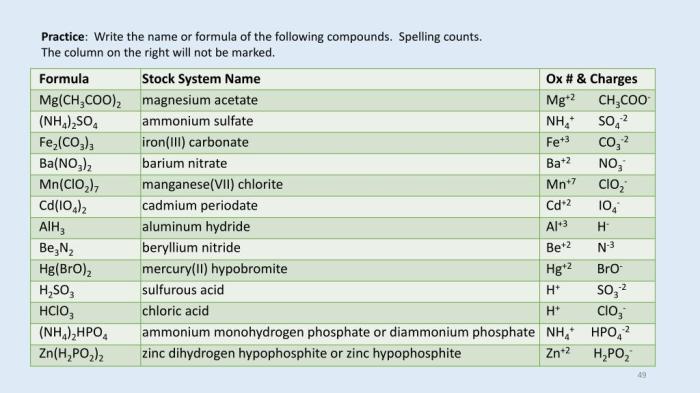
For chemistry buffs, you might know that the chemical name for MgH2PO3.2 is magnesium hydrogen phosphate. Speaking of legal cases, have you heard of the Luthi v. Evans case brief ? It’s an interesting read. Anyway, back to chemistry, MgH2PO3.2
is a salt that can be used in various applications.
Physical Properties
MgH2PO3·2H2O is a white, crystalline powder that is soluble in water. It has a melting point of 110°C.
Chemical Properties: Mg H2po3 2 Chemical Name
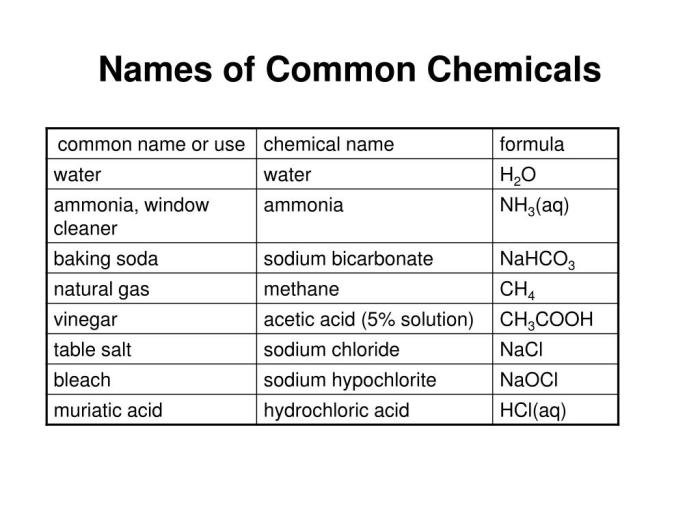
Magnesium hydrogen phosphate dihydrate (MgH 2PO 3·2H 2O) exhibits both acidic and basic properties due to its amphoteric nature.
Acidity and Basicity
MgH 2PO 3·2H 2O reacts with both acids and bases, demonstrating its amphoteric nature. When it reacts with a strong acid like hydrochloric acid (HCl), it acts as a base and forms magnesium chloride (MgCl 2) and phosphoric acid (H 3PO 4):
MgH2PO 3·2H 2O + 2HCl → MgCl 2+ H 3PO 4+ 2H 2O
Conversely, when MgH 2PO 3·2H 2O reacts with a strong base like sodium hydroxide (NaOH), it acts as an acid and forms magnesium sodium phosphate (MgNaPO 3) and water (H 2O):
MgH2PO 3·2H 2O + NaOH → MgNaPO 3+ 3H 2O
Reactions with Other Chemicals
MgH 2PO 3·2H 2O reacts with various other chemicals. For example, it reacts with silver nitrate (AgNO 3) to form a white precipitate of silver phosphate (Ag 3PO 4) and magnesium nitrate (Mg(NO 3) 2):
3MgH2PO 3·2H 2O + 6AgNO 3→ Ag 3PO 4+ 2Mg(NO 3) 2+ 6H 2O
Thermal Stability
MgH 2PO 3·2H 2O is thermally stable up to approximately 100°C. Upon heating beyond this temperature, it undergoes dehydration and decomposition to form magnesium pyrophosphate (Mg 2P 2O 7) and water:
2MgH2PO 3·2H 2O → Mg 2P 2O 7+ 4H 2O
Applications

Magnesium hydrogen phosphate dihydrate (MgH2PO3·2H2O) finds diverse applications in various industries, including agriculture, medicine, and emerging technologies. Its unique properties make it a valuable compound for specific purposes.
Industrial Applications, Mg h2po3 2 chemical name
In the industrial sector, MgH2PO3·2H2O is utilized as a fire retardant and flame retardant in the production of textiles, plastics, and other materials. It acts as a flame retardant by releasing water vapor and non-combustible gases, which helps to suppress the spread of fire and reduce the risk of combustion.
Agricultural Applications
In agriculture, MgH2PO3·2H2O is employed as a fertilizer to provide magnesium and phosphorus to plants. Magnesium is essential for chlorophyll production, photosynthesis, and enzyme activation, while phosphorus plays a crucial role in root development, energy transfer, and cell division. By supplying these nutrients, MgH2PO3·2H2O enhances plant growth and productivity.
Medical Applications
In the medical field, MgH2PO3·2H2O is used as an antacid to neutralize stomach acid and alleviate heartburn, indigestion, and other digestive issues. It works by reacting with stomach acid to form magnesium salts, which have a buffering effect and help to restore the pH balance in the stomach.
Emerging Technologies
Beyond traditional applications, MgH2PO3·2H2O is being explored for potential uses in emerging technologies. For instance, it is investigated as a potential electrolyte material in solid-state batteries due to its high ionic conductivity and thermal stability. Additionally, its ability to release hydrogen gas upon heating makes it a promising candidate for hydrogen storage and energy applications.
Safety and Handling
Magnesium hydrogen phosphate dihydrate (MgH2PO3·2H2O) requires careful handling to prevent potential hazards. It is essential to follow safety precautions to minimize risks associated with its use.
Potential Hazards
- Eye and Skin Irritation:Exposure to MgH2PO3·2H2O can cause irritation to the eyes and skin. Direct contact should be avoided.
- Inhalation:Inhaling MgH2PO3·2H2O dust or mist can lead to respiratory irritation, coughing, and shortness of breath.
- Ingestion:Ingesting MgH2PO3·2H2O can cause gastrointestinal discomfort, nausea, and vomiting.
Safety Precautions
- Protective Gear:Wear appropriate protective gear such as gloves, safety glasses, and a dust mask when handling MgH2PO3·2H2O.
- Ventilation:Ensure adequate ventilation in areas where MgH2PO3·2H2O is being used or stored.
- Avoid Contact:Minimize direct contact with MgH2PO3·2H2O by using appropriate tools and techniques.
- Emergency Response:In case of exposure, flush affected areas with plenty of water and seek medical attention if necessary.
Storage and Disposal
MgH2PO3·2H2O should be stored in a cool, dry place in a tightly sealed container. Keep away from incompatible materials such as strong acids and bases. Dispose of MgH2PO3·2H2O according to local regulations for hazardous waste.
Common Queries
What is the chemical formula of MgH2PO3·2H2O?
MgH2PO3·2H2O
What is the molar mass of MgH2PO3·2H2O?
123.31 g/mol
What is the appearance of MgH2PO3·2H2O?
White, crystalline
What is the solubility of MgH2PO3·2H2O in water?
High
What is the melting point of MgH2PO3·2H2O?
100°C