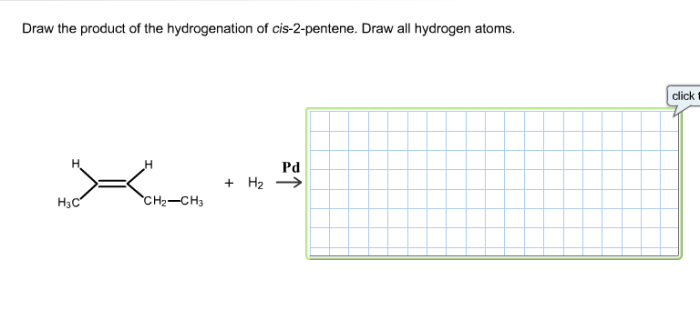
Design a synthesis of m-bromostyrene from benzene is a fundamental reaction in organic chemistry that involves the electrophilic aromatic substitution of benzene with bromine. This reaction is widely used in the synthesis of various aromatic compounds, including pharmaceuticals, dyes, and fragrances.
In this article, we will delve into the detailed mechanism, optimization, and characterization of this important reaction.
The bromination of benzene is a regiospecific reaction, yielding predominantly m-bromostyrene as the product. This regioselectivity is attributed to the directing effect of the bromine substituent, which activates the meta position of the benzene ring towards electrophilic attack. The reaction proceeds via an electrophilic aromatic substitution mechanism, involving the formation of a Wheland intermediate and subsequent rearomatization.
Synthesis of m-Bromostyrene from Benzene
The synthesis of m-bromostyrene from benzene is a fundamental organic reaction in the field of aromatic chemistry. This reaction involves the electrophilic aromatic substitution of benzene with bromine (Br2) to selectively produce m-bromostyrene, a valuable intermediate in the synthesis of various pharmaceuticals, dyes, and polymers.
Benzene Properties and Reactivity: Design A Synthesis Of M-bromostyrene From Benzene
Benzene, a six-membered aromatic hydrocarbon, possesses unique chemical properties due to its resonance-stabilized structure. The delocalized π-electrons within the benzene ring confer aromatic stability, making it less reactive towards addition reactions and more susceptible to electrophilic aromatic substitution.
Electrophilic Aromatic Substitution Reaction Mechanism, Design a synthesis of m-bromostyrene from benzene
Electrophilic aromatic substitution involves the attack of an electrophile (a species seeking electrons) on the benzene ring. The electrophile adds to one of the carbon atoms in the ring, forming a new bond and disrupting the aromaticity. The resulting intermediate then undergoes rearomatization to restore the aromatic stability.
Bromination of Benzene
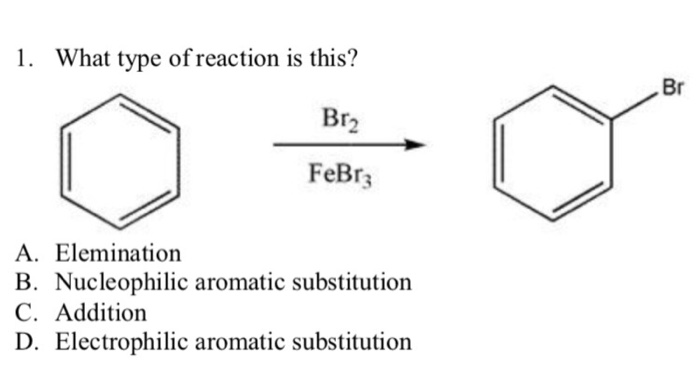
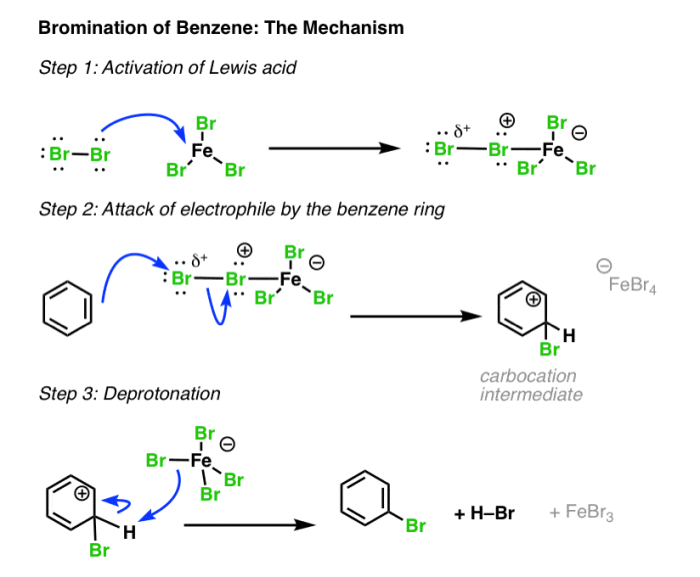
The bromination of benzene is a classic example of electrophilic aromatic substitution. In this reaction, bromine (Br2) acts as the electrophile, and a Lewis acid catalyst, such as aluminum bromide (AlBr3), is used to activate the bromine molecule.
The catalyst coordinates with one of the bromine atoms, weakening the Br-Br bond and making the bromine molecule more electrophilic. The activated bromine molecule then attacks the benzene ring, forming a bromonium ion intermediate. This intermediate subsequently undergoes rearomatization to yield m-bromostyrene.
Regioselectivity of the Reaction
The bromination of benzene exhibits regioselectivity, meaning that the bromine atom preferentially substitutes at the meta (m) position of the benzene ring. This regioselectivity is attributed to the steric hindrance caused by the methyl group in styrene, which directs the incoming electrophile to the less hindered meta position.
Mechanism of Electrophilic Aromatic Substitution
The mechanism of electrophilic aromatic substitution can be summarized as follows:
- Electrophile Formation:The Lewis acid catalyst activates the electrophile by coordinating with it and weakening its bond.
- Attack on the Benzene Ring:The activated electrophile attacks the benzene ring, forming a new bond with one of the carbon atoms and disrupting the aromaticity.
- Rearomatization:The intermediate formed in step 2 undergoes rearomatization by expelling a proton (H+), restoring the aromatic stability of the benzene ring.
Optimization of Reaction Conditions
The yield and selectivity of the bromination reaction can be optimized by controlling various reaction conditions:
- Temperature:Higher temperatures favor the formation of m-bromostyrene by promoting the rearomatization step.
- Solvent:Polar solvents, such as dichloromethane, enhance the solubility of the reactants and facilitate the reaction.
- Catalyst Concentration:Increasing the catalyst concentration accelerates the reaction by providing more activated electrophile.
Isolation and Purification of m-Bromostyrene
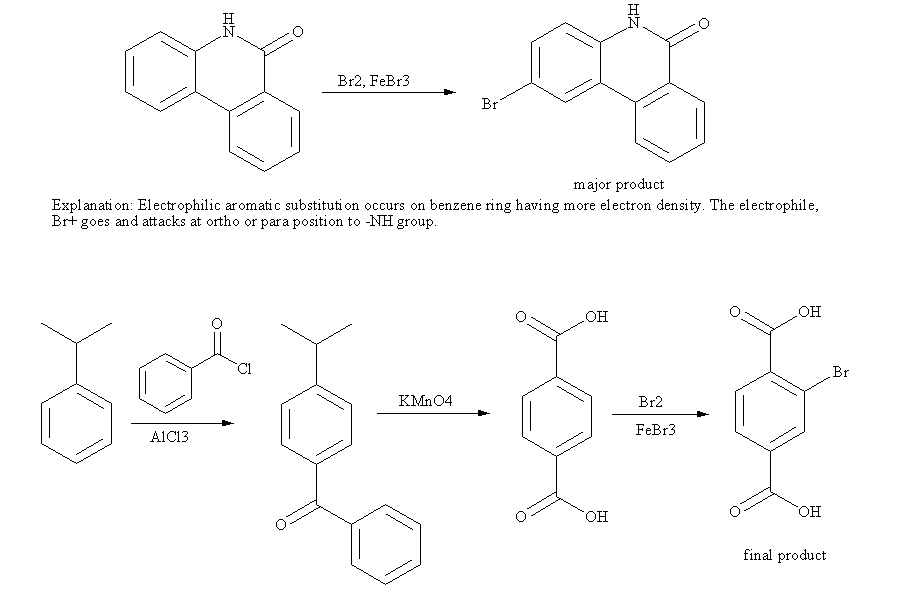
After the reaction is complete, m-bromostyrene can be isolated and purified using the following techniques:
- Extraction:The reaction mixture is extracted with an organic solvent, such as diethyl ether, to separate the product from the aqueous phase.
- Distillation:The organic extract is distilled to remove the solvent and other volatile impurities.
- Crystallization:The distilled product can be further purified by crystallization from a suitable solvent, such as ethanol, to obtain pure m-bromostyrene.
Characterization of m-Bromostyrene
The structure and purity of m-bromostyrene can be confirmed using various spectroscopic techniques:
- NMR Spectroscopy:1H and 13C NMR spectroscopy can provide information about the structure and connectivity of the molecule.
- IR Spectroscopy:IR spectroscopy can identify the presence of functional groups, such as the C-Br bond.
- Mass Spectrometry:Mass spectrometry can determine the molecular weight and elemental composition of the product.
FAQ Guide
What is the mechanism of the bromination of benzene?
The bromination of benzene proceeds via an electrophilic aromatic substitution mechanism, involving the formation of a Wheland intermediate and subsequent rearomatization.
What factors affect the yield and selectivity of the bromination reaction?
The yield and selectivity of the bromination reaction are affected by factors such as temperature, solvent, and catalyst concentration.
How is m-bromostyrene isolated and purified?
m-Bromostyrene is isolated and purified from the reaction mixture using techniques such as extraction, distillation, and crystallization.